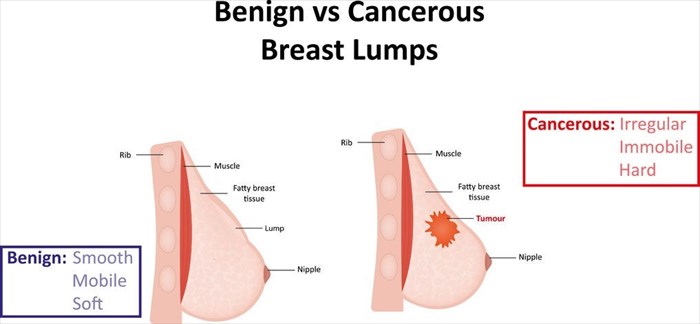
We all know the importance of screening for breast cancer and the various factors which increase a person’s risk of developing it. With such high prevalence rates, the standard practices for treatment are also something most of us, at the very least, have an idea about. Even if all you have is a vague idea, generally you’ll know that breast cancer treatment is invasive and often, debilitating. What if there was an alternative option which could be even more effective?
Quick facts about breast cancer
- Breast cancer is one of the most common forms of the disease occurring in women across the world, with more than a million new cases diagnosed every year – that’s approximately 25% of all diagnosed cancer cases.
- The 2012 GLOBOCAN (global cancer project) report released in 2012 estimated a total of 521,907 fatalities as a result of breast cancer during the same year. (1) General mortality rates in developed countries have declined considerably with earlier detection and treatment (prevention and control strategies) initiated by numerous awareness drives, educating more women regarding risk factors.
- Risk factors include:
- A personal history of malignant or benign tissues
- A family history of breast cancer (specifically in a first-degree relative i.e. a parent or sibling)
- BRCA1 and BRCA2 (breast cancer susceptibility genes) gene mutations (or changes)
- Dense breast tissue
- Breast tissue exposure to oestrogen (which is associated with early age menstruation, late onset of menopause, first pregnancy and birth over the age of 30 or never bearing any children)
- The taking of hormone medications combining oestrogen and progestin / progesterone (such as for oral contraceptive pills or those used in the treatment of menopause). Many breast cancer subtypes are associated with hormonal problems.
- The World Cancer Research Fund International estimates that early stage breast cancer survival rates in countries with advanced medical care is between 80 and 90%. Breast cancer that is diagnosed at an advanced or late stage may only achieve as much as a 24% survival rate following treatment. (2)
Cryoablation, a breast tumour treatment option
Therapies which are minimally invasive are appealing to both patients and many medical professionals. They form part of a growing trend in the medical field too. More and more experts and patients alike are calling for the development of less invasive therapies, which are also cost effective for global health sectors.
Invasive procedures are currently the gold standard of treatment – they are often complex and come with a range of risk factors. Pain, discomfort, bleeding, infection risk… the list can go on. There are no guarantees that a patient will be cancer-free at the end of such an experience either.
For many individuals, treatment recommendations involve a mastectomy – the surgical removal of breast tissue. Removal can either be a partial or complete extraction of either one or both breasts. Axillary lymph node dissection (the surgical removal of lymph nodes) is also sometimes performed should malignancy have begun to spread.
Recovery from such a procedure requires downtime ranging between several days to a few weeks (depending on a woman’s condition), and for many, may be followed up with rounds of radiation therapy or chemotherapy to ensure that no trace of cancer is left in the body. These therapies are laden with challenges and adjustment difficulties. Simply put, treatment is not a comfortable or pleasant experience and challenges anyone’s sense of dignity.
If breast cancer patients could opt to forgo the knife or the sickening effects of chemo and try another form of targeted tumour treatment, there’s a strong chance that many with a suitable candidate profile would.
Research has looked at a variety of options offering less invasive means of treating malignant tumours and medical experts have continuously been making headway. The question of ‘what if’ is now strongly moving into an area of ‘it’s possible’… Trials are actively in progress, testing the various factors necessary to be taken into consideration before any options can be deemed viable and safe enough to be offered as part of standard practice. Researchers are working towards achieving just that. For patients to be able to have another effective choice is important. For medical professionals to be able to offer another option that is at the very least on par with existing ones, or could potentially achieve better results, is important too. Everyone involved could stand to benefit if this could be achieved.
One such example of a potentially effective therapy option is that of the IceSense3™ system, developed by IceCure Medical Ltd. The system has undergone some extensive research in a bid to prove that low risk, early stage malignant tumours can be successfully treated without the need for surgical extraction. The system thus allows for an alternative procedure which could be considered as a viable replacement for surgery in many patients. Recovery and downtime are virtually done away with due to the minimally invasive techniques employed in targeting tumours for treatment. It isn’t too good to be true either…
Who are IceCure Medical?
Founded in 2006, IceCure Medical is a U.S based medical device company that both develops and markets minimally invasive therapy systems for the improvement of women’s health. The company has given particular focus to cryoablation therapies and others pertaining to the general oncology market. The objective is to develop therapies which are as safe as they are effective, thus optimising the chances of a return to a healthy physical state. Improved technology which results in improved preservation of life is paramount for this organisation, who have been able to successfully demonstrate such a possibility.
Earlier this year, IceCure Medical shared updated findings from an ongoing body of research aimed at applying cryoablation technological procedures to malignant (cancerous) tumours. Cryoablation has been in use for years for non-cancerous varieties. This technique is currently an FDA (U.S. Food and Drug Administration) approved treatment application capable of effectively destroying benign (non-cancerous) breast tissue or tumours (fibroadenomas). Approval was granted in 2002 following extensive and successful research findings. It is also CE certified within the EEA (European Economic Area).
Research has gone a step further, having seen substantial success in treating fibroadenomas, which are fairly common in many women, developing most often during puberty (although they can occur at any age). Much research has been conducted looking into whether cryotherapy could indeed be as viable a treatment option for malignant tumours.
IceCure Medical began a national multi-center clinical trial in the US using cryoablation (ICE3) as its treatment modality (for non-surgical breast cancer treatment) in 2014. This ongoing trial has already achieved more than satisfactory results and continues to impress those in medical circles as new information is compiled and released. Through years of careful study, it would seem that such a procedure is continuously making strides in the right direction. It could very well become an available option for doctors and patients alike around the world in the near future.
What is cryoablation?
Cryoablation is a technique whereby extreme cold is targeted at tumours with the aim of destroying the bodily tissues which they are comprised of. Extreme cold is delivered to these tissues with the use of hollow needles (probes), through which thermally conductive (cooled) fluids (liquid nitrogen) can be administered.
Cryoablation as a technique is the original ablative approach (there are various others used for different purposes) and was the first to be considered for breast cancer research purposes. The first ever attempt using such a technique was conducted using a mouse model in 1985 and a 70% reduction in tumour recurrence was achieved. (3) In the decades that have followed, the idea that cryoablation could be used for targeted tumour treatment, including malignant variations has continued to be explored. In humans, the technology has been in use for more than 20 years and has formed part of at least 7 clinical studies. (4) Researchers continue to explore the potential of this technique, and it would seem that it is now becoming a promising option which could soon be considered a viable first-line treatment alternative to the currently preferred surgical route – in patients with low risk, small tumours that is.
Cryoprobe technology has gone through ‘generation’ adjustments over the years, improving the tools intended for use. The first applicators or probes used were cooled with nitrogen. Second generation applicators were thermoelectric. Now, refrigerated applicators have been developed. The probes in use today are thinner and capable of performing a procedure that is near painless and negates the need for general anaesthesia and incisions.
The use of cold temperatures has an analgesic effect, helping to numb the treatment area along with an injected anaesthetic (such as lidocaine). All a patient (who is merely under light sedation and is not unconscious at all) is likely to feel during the treatment is a sensation of coolness. The procedure is cosmetically appealing too – there is no need for sutures (stitches) to be made and very little post-procedural scarring. For many, the tiny scar that forms where the thin probe pierces the skin becomes virtually invisible once healed. The destruction of the tumour will also not change the shape or size of the breast following treatment. No deformity of the breast occurs when treating small tumours.
The technology which is currently being used in benign tumour treatment and that which is being tested for malignant tumours can be used to effectively perform procedures in a medical office environment with the patient under local anaesthetic. This is another appealing factor – no need for hospital administrations and the complication risks associated with general anaesthesia. Added bonuses are that the procedure is less expensive to perform than surgical options, has little down time and also provides a patient with a better cosmetic outcome. No medications to manage pain are generally required following treatment either. The procedure is expected to be useful in treating symptomatic tumours located in other portions of the body too, including organs like the prostate, lungs, kidneys or liver. Research is also looking at the effectiveness of this technique in treating cancer affecting bone structures.
So how does the basic cryoablation procedure work?

The probe is designed with vacuum insulation along its entire length. This enables better protection of the tissues and skin surrounding the targeted treatment zone / tumour. The probe also has a small disposable component which allows a new one to be used for each patient. The console portion of the system is designed to include a touch screen which a doctor can use to help customise a treatment, tailoring the procedure for individual patients. A treating doctor may use the console touch screen, handle, foot pedal or buttons on the handle to control the system during the procedure.
Through years of study, researchers have noted that cryoablation as a technique is effective for tumours when they become clinically problematic. At this stage many of these tumours, whether benign or malignant, measure between 1 and 3 cm in diameter. Cryoablation as a technique has been seen to effectively treat such size tumours, especially for those measuring between 1 and 2cm. Success rates have been highest for those measuring 1.5cm or less, including those that are malignant. (5)
One of the key factors that makes this procedure minimally invasive is that it is image-guided. The applicator is guided with the use of ultrasound or computerised tomography (CT) imaging scan. No extractions take place. Instead, the probe / applicator is inserted and targeted towards the centre of the tumour with the use of imaging. Liquid nitrogen is inserted into the hollow applicator, which directs the flow of the substance to the targeted tumour tissue.
The cold temperature of the probe / applicator and liquid nitrogen are then used to ‘ablate’ (i.e. destroy) the tumour within its original location.
Essentially, the procedure is conducted in three phases:
- Freezing
- Thawing
- Freezing
Freezing cycles can take between 5 and 8 minutes each with a short period of thawing in between. This is what initiates tumour cell death.
This three-phase approach promotes tumour destruction in four ways:
- Freezing via the use of liquid nitrogen encourages the formation of ice crystals which disrupt the tumour cell membrane
- Osmotic dehydration (loss of fluid) results in the coagulation (clotting) of blood
- This then causes a restriction of blood supply to the tissues (ischemia) and…
- A defence reaction (immunologic response) is initiated whereby antibodies are produced by the body in order to protect it from the antigen or foreign substance being introduced (i.e. the liquid nitrogen).
Ultimately the three-phase process stimulates an ‘anti-cancer’ immunologic response, helping to rid the body of malignant cells.
A warming cycle takes place when the physician is ready to extract the applicator or probe from the breast.
About the destruction process, chief of surgical oncology at the University of Michigan Hospital, Dr Michael Sabel said that “Cryoablation makes the cells burst and they release all that cancer DNA into the system. The body's immune system almost acts as an auto-vaccine against the cancer.”
By ablating the source of cancer (i.e. the malignant tumour from which the disease originated) an individual’s potential for metastatic advancement (i.e. the spreading of cancer) is eradicated too. The researchers believe that this may very well reduce the possibility of cancer spreading to other parts of the body, as well as any odds of a recurrence.
The targeted tumour that is treated with the procedure will gradually reduce in size in the months that follow. An individual undergoing such a procedure may thus still be able to ‘feel a lump’ for a period of time after the treatment – a treating doctor will likely ensure that a patient is aware of this beforehand.
The procedure does not offer an instant obliteration of the tumour – it merely initiates the ultimate desired effect, shrinkage and eventual disappearance of the tumour takes some time. A follow-up with the treating medical doctor will determine whether or not the procedure has been successful in eradicating the malignant tumour. By successful, we mean a progressive reduction in size until the tumour is no longer existent. The tumour reduces in size as the body reabsorbs the destroyed tissues in the months following treatment. At 6 months, a tumour can be reduced by at least 40% and close to 90% by 12 months following treatment. Follow-ups will include mammograms and other imaging scans like sonars to confirm successful treatment – with the end goal being a clear scan showing no further signs of the tumour or cancerous cells.
For a medical doctor, the procedure allows for precision targeting of the tumour without the need to surgically remove it from the body. As the ice-ball forms, it can clearly be seen with the visual devices used.
The process is not tremendously time-consuming, and can be done in well under an hour, sometimes even as quickly as 20 minutes. A patient is able to resume daily responsibilities without the need for downtime or prolonged recuperation. Once the ablation procedure is complete, the small skin nick (about 3mm) where the applicator / probe was inserted is covered with a sterilised gauze adhesive bandage.
The ICE3 clinical trial
The breast is an area of the body that is seemingly well-suited to ablative techniques. Hence, it is one area researchers have strongly concentrated on when conducting extensive studies. Breast tissue is covered by the skin without any intervening structures. Medical professionals can thus access breast tissue effectively and easily image the structure with the use of scan technology like ultrasound.
In 2014, a multi-centred clinical trial for low-risk small breast tumour malignancies was initiated within 18 selected medical sites (clinics and hospitals) around the United States, including Mount Sinai Beth Israel, Weill Cornell Medical College, Montefiore Medical Center and Columbia University Medical Center in New York. (6) Other sites were located around California, Arizona, Connecticut, Indiana, Georgia, Michigan, New Mexico, New Jersey, Ohio, Pennsylvania, Tennessee and Texas.
These sites were selected as they already had experience using the IceSense3 console devices in the treatment of fibroadenomas. The first patients were enrolled and efficacy testing of cryoablation commenced. This trial is the largest controlled study for breast cancer tumour treatment. By October of 2014, IceCure Medical announced successful results for their first two participating patients. The medical researchers involved felt confident that their trial was on the right track to expand on the technique’s already recorded data and perhaps prove to be a viable first-line treatment option for some breast cancer patients.
At the time, one of the participating medical professionals, Dr Linsey Gold said that their success using cryoablation “promises many clinical and quality of life benefits for patients. We hope to prove them in this study.”
Dr Richard Fine, from The West Clinic in Memphis, Tennessee – also involved in the trial, added that “Improved screening allows physicians to identify breast cancer earlier… Advances in molecular profiling help us better determine which breast cancers have higher or lower risks of recurrence. We can then individualise approaches to treatment. Now with the ICE3 study, we may have the first effective non-surgical treatment option for some low risk patients.”
In May this year, IceCure Medical announced impressive results from their ongoing trial – 99% of malignant breast tumours in participating patients were successfully destroyed using cryoablation. Updated data along with this incredible result was presented by representatives of the trial at the ASBS (American Society of Breast Surgeons) conference, held in Orlando, Florida (United States). For the many medical decision makers and surgeons attending this conference from all over the world, freezing malignant breast tumours appeared to be a strong alternative treatment option to take note of. It’s not just a potential idea anymore. It’s working.
The trial has thus far recruited 200 women over the age of 50, all with diagnosed low-risk breast cancer (i.e. early stage). Each woman had tumours measuring 1.5cm in diameter. Each participating woman was treated with the IceSense3 console system. Procedures ranged between 20 and 40 minutes without the need for hospitalisation. All women were able to return home after a short period following their procedure. Around 76% of the participants were able to comfortably resume daily activity within the initial 48 hours following their treatment.
Follow-ups for each participant will take place every 6 months during the next 5 years. The goal of the trial is to achieve complete ablation of malignant tumours for 60 months (5 years) following cryoablation treatment. Annual check-ups will be required for all participants after the initial 5 years to monitor their condition.
Only one of the 200 study participants experienced a recurrence (which was able to be treated with an alternative standard therapy) and the majority of participating women are actively in a follow-up period stage of close to 2 years since their initial treatment – with no indications of malignant tissue recurrence. To date, none of the participants have experienced significant side-effects or complications either. The most common include transient discomforts like swelling, mild pain, bruising and tenderness. Adhesive tape blisters or the formation of keloid scars can also occur. Researchers conducting the trial reported that as many as 95% of the participating women were satisfied with the cosmetic results following their treatment.
Eyal Shamir, CEO at IceCure Medical is also extremely satisfied with the trial results, "The treatment with IceSence3™ provides a simple, precise and safe alternative to surgical procedures for removing the cancerous tumour in the breast. Treatment with our system enables the elimination of the entire tumour by freezing. Patients taking part in the trial can enjoy their routine lives after a brief and straightforward treatment."
The trial is ongoing and is estimated to be completed by December 2024. So far, the results appear highly successful. There is a growing interest in developing more medical therapies, wherever possible, which can be successfully minimally invasive, more cost effective for health sectors and cosmetically appealing. The IceSense3 cryoablation technique is certainly one which has demonstrated highly, promising efficacy. This procedure may just be a handful of steps away from becoming a viable option for many doctors and their patients with early stage breast cancer.
Dr Rache Simmons, chief of breast surgery at the Weill Cornell Medical Center and one of the study’s authors believes that cryoablation is “… a huge advance for women. I think this could be the wave of the future.”
If made more widely available for breast cancer patients, can you just imagine the significant effect cryoablation could have on the disease and the lives it affects? We look forward to the next updates and hope that the successes achieved thus far continues.
References:
1. International Agency for Research on Cancer. 2012. Globocan 2012 Cancer Fact Sheets - Breast Cancer: http://gco.iarc.fr/today/fact-sheets-cancers?cancer=15&type=0&sex=2 [Accessed 03.08.2018]
2. World Cancer Research Fund International. 2015. Breast Cancer Statistics: https://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics [Accessed 03.08.2018]
3. US National Library of Medicine - National Institutes of Health. June 2014. Percutaneous Image-Guided Ablation of Breast Tumors: An Overview: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4078155/ [Accessed 03.08.2018]
4. US National Library of Medicine - National Institutes of Health. January 2016. A review of ablative techniques in the treatment of breast fibroadenomata: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4717637/ [Accessed 03.08.2018]
5. US National Library of Medicine - National Institutes of Health. June 2014. Percutaneous Image-Guided Ablation of Breast Tumors: An Overview: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4078155/ [Accessed 03.08.2018]
6. ClinicalTrial.Gov. 25 July 2014. Cryoablation of Low Risk Small Breast Cancer- Ice3 Trial: https://clinicaltrials.gov/ct2/show/NCT02200705 [Accessed 03.08.2018]