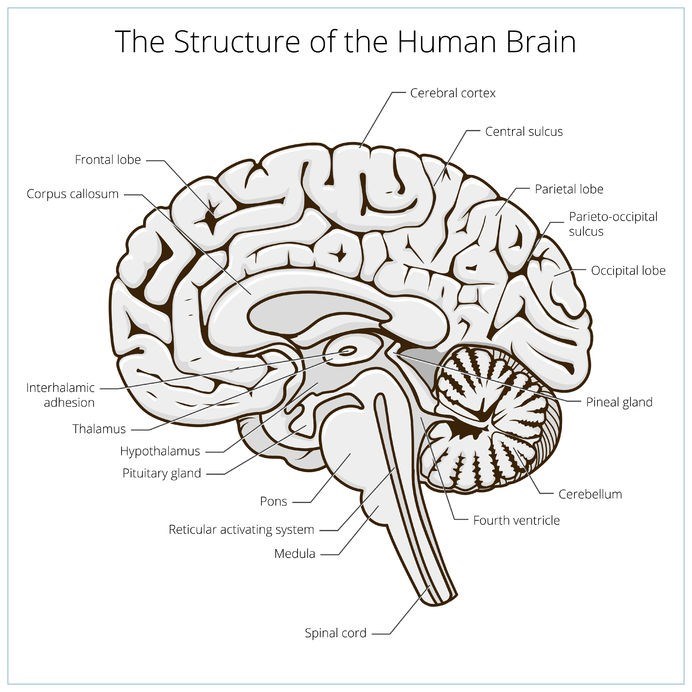
- Chronic Traumatic Encephalopathy (CTE)
- Neuropathological stages of Chronic Traumatic Encephalopathy (CTE)
- Signs and symptoms of Chronic Traumatic Encephalopathy (CTE)
- How is Chronic Traumatic Encephalopathy (CTE) diagnosed?
- Is Chronic Traumatic Encephalopathy (CTE) treatable?
- How can Chronic Traumatic Encephalopathy (CTE) be prevented?
Neuropathological stages of Chronic Traumatic Encephalopathy (CTE)
Neuropathological progression stage characteristics (7)
Through ongoing research and based on what is currently known about the condition and its pathological patterns so far, chronic traumatic encephalopathy (CTE) is classified according to four different stages. Each stage is characterised according to pathological progression and an associated set of clinically recognised symptoms.
| Stage I | Stage II | Stage III | Stage IV | |
| P-tau accumulation pattern | Focal Neurofibrillary Tangles (i.e. twisted fibres inside the brain's cell thought to contribute to the degradation of the brain’s nerve cells which are located in a single (localised) area of the brain) and neuropil neurites (myelinated nerve fibres) are located within the grooves (sulci) of the superior and dorsolateral superior frontal, and temporal or septal cortex (i.e. in the depths of the fissures in these areas of the brain). Accumulations of p-tau (a protein that stabilises structures within the central nervous system in healthy individuals, but when these structures are damaged, it is released into the nerve cells of the brain, causing a build-up that results in clumps in them. The accumulation results in damage to the brain cells which affects brain function) are characterised by their location in isolated centres around the blood vessels. |
Multiple Neurofibrillary Tangle (NFT) clusters and neuropil neurites (i.e. a dense network of interwoven nerve fibres and the projections from the cell bodies) are mostly found in the frontal, temporal, parietal, insular and septal cortices of the brain. Some may also form adjacent to the central areas and within the nucleus basalis of Meynert (a group of nerve cells situated in the basal forebrain) and the locus coeruleus (where norepinephrine, the chemical released by the nervous system in response to stress is synthesised). Here, NFT degeneration can occur which affects nerve cell networks in the brain. |
Moderately dense accumulations of NFTs form in the medial temporal lobes and become more widespread – affecting the spinal cord, brain stem and diencephalon (a part of the brain situated at the upper end of the brain stem, between the cerebrum and the brain stem). NFT degeneration is notable in the medial temporal lobe structures – the hippocampus (the area of the brain that regulates emotions), amygdala (a portion of the brain that plays a key role in processing emotions) and entorhinal cortex (this acts as a hub in the brain’s vast network of memory and navigation). Moderate accumulations of p-tau may also be present in the olfactory bulbs (areas responsible for interpreting smells detected by the nose), thalamus (the area of the brain with multiple functions including the regulation of consciousness, sleep and alertness as well as relaying sensory signals), hypothalamus (which plays a role in releasing hormones and regulating body temperature), mamillary bodies (the front part of the thalamus involved in recollective memory function), substantia nigra (an area that plays a role in reward, addiction and movement), as well as the dorsal and median raphe nuclei (which release serotonin to the rest of the brain). |
NFTs / p-tau accumulation is widespread and dense - including formation in the white matter of the brain (forming white matter lesions / WMLs). Accumulation of p-tau is most advanced and severe at this stage and can also be found in the cerebellum, brain stem and diencephalon portion of the brain, which contains the epithalamus, thalamus, hypothalamus, ventral thalamus and third ventricle. Protein formations result in prominent nerve cell loss (known as hippocampal sclerosis) and damage to the glial cells (gliosis) within the cerebellum / cortex. This damage may cause symptoms similar to those exhibited in dementia and Alzheimer’s Disease (such as memory loss, cognitive difficulty, issues with speech etc). |
| Macroscopic changes (i.e. changes that are visible to the naked eye in imaging tests like MRIs) | Mild enlargement of the lateral ventricles (the two largest cavities in the human brain) may occur. | Mild enlargement of the lateral ventricle frontal horn occurs. Enlargement of the third ventricle or small cavum pellucidum (located near the midline of the brain) may also occur. | Mild cerebral atrophy (cell degeneration) occurs whereby ventricles become further enlarged, the locus coeruleus and substantia nigra experience depigmentation (the loss of pigment resulting in these structures appearing white) as the nerve cells that contain a dark pigment (called melanin) die and septal abnormalities develop. | Cerebral atrophy (a loss of nerve cells and the connections between them in the brain) increases, affecting the medial temporal lobe, hypothalamus, thalamus and mammillary body. Septal irregularities develop (such as cavum septum pellucidum), ventricles become further enlarged and further abnormalities (i.e. depigmentation resulting in pallor) in the locus coeruleus and substantia nigra develop. |
| TDP-43 (TAR DNA-binding protein 43) – encoded by the TARDBP gene This is essentially a mutation caused in a gene that is linked with the development of CTE. |
In the initial stages the presence of TDP-43 (dot-like thread inclusions of this protein) in the cerebral cortex, medial temporal lobe and brain stem (occurring around the blood vessels (perivascular), ventricles (periventricular) and beneath the membranes surrounding the brain (subpial)) is noted. | TDP-43 occurs in the cerebral cortex, medial temporal lobe and brain stem. During these stages inclusions (accumulations of this protein) are characterised as cytoplasmic neuronal entities, this means deposits of the protein are contained within the jelly-like substances that make up the brain’s nerve cells (neurons). | The presence of TDP-43 protein in the same areas where p-tau is present results in severe intraneuronal and intraglial inclusions (accumulations of these proteins within the nerve and glial cells), located in the cerebral cortex (the area of the brain involved in consciousness, thinking, understanding and speaking), white matter, diencephalon, basal ganglia and brain stem. | |
| Nerve fibre injury / damage | Presynaptic terminals (the transmitting units of nerve cells) develop along unmyelinated nerve fibres (i.e. nerve fibres not covered with myelin which forms an electrical insulating layer that helps with communication). This results in multifocal (i.e. occurring in multiple regions of the brain) / distorted nerve fibre swellings / bulges (varicosities) in the cerebrum / cortex, as well as the subcortical white matter of the brain which ultimately result in the disconnection of nerve fibres, rendering communication signals between nerve cells ineffective. |
Multifocal nerve fibre bulges (varicosities) continue to occur (progressively worsening) in the cortex and subcortical white matter, further inhibiting communication signals. | A severe loss of nerve fibres begins to occur within the cortex and white matter of the brain. Loss is most severe in the frontal and temporal lobe regions which control cognitive function (emotions, memory, language, problem solving, judgment, and sexual behaviour) and hearing / sound processing respectively. This effectively results in the sufferer experiencing issues in these areas. |
Nerve fibre loss continues to severely affect the cortex and white matter areas of the brain. Distorted nerve fibre swellings (varicosities) are widespread and the symptoms experienced in the previous stage intensify. |
Accumulations (or clumps) of beta-amyloid (Aβ) peptides (or Aβ plaques) – deposits of Aβ1–42 have been found in some officially diagnosed CTE cases and appear to be most significant in older patients with progressed stages of the condition. It does not appear to be a major characteristic of early stage CTE. When these plaques or clumps build up, they may also contribute to the blockage of cell to cell signalling which affects the functions of the area of the brain they are located in.
Reference:
7. Handbook of Clinical Neurology - Third Series: Traumatic Brain Injury - Part 1. 2015. The Neuropathology of Traumatic Brain Injury: https://books.google.co.za/books?id=LVl3BAAAQBAJ&pg=PA55&lpg=PA55&dq#v=onepage&q&f=false [Accessed 10.05.2018]